New Dry Eye Treatment 2016
New dry eye treatment 2016. Potential new treatment for severe dry eye disease. These are interesting approaches although we need more studies to establish their safety and efficacy over the long term he said. Allergan recently beefed up its sales force DTC advertising and.
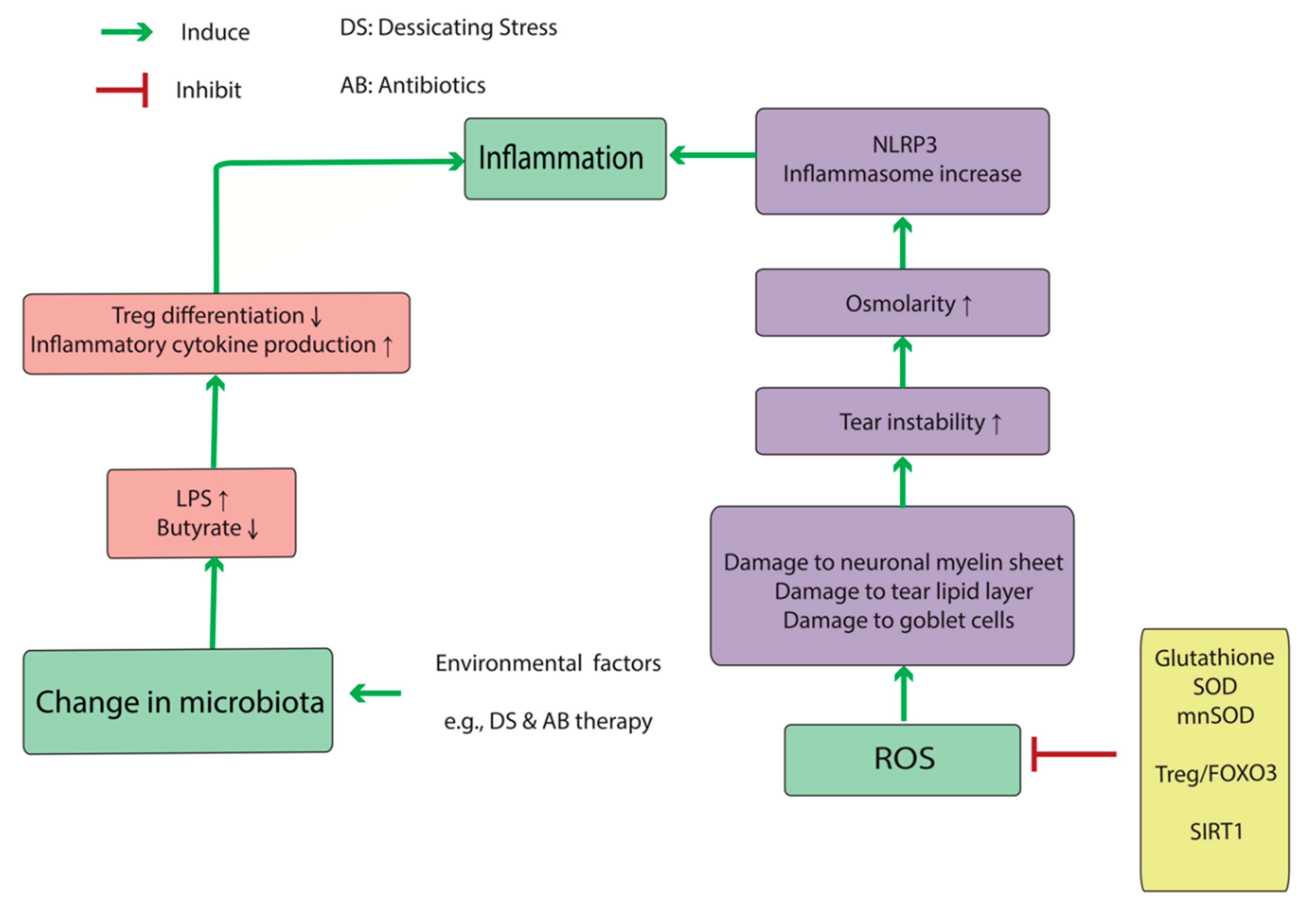
While the proliferation of dry eye pharmaceutical agents has been sluggish the dry eye device market has not. Treatment will be different for each person depending on the root cause of the dry eye. DNase is currently approved by the US.
Some of these procedures require a series of treatments to achieve results. The first and only medication approved by the US FDA for the treatment of both signs and symptoms of dry eye this novel eyedrop was developed specifically for the eye by Shire a global leader in pharmaceuticals. 1 is a treatment concept for dry eye recommended by the Japanese Dry Eye Society and Asia Dry Eye Society Tsubota et al 2017.
CF101 is an anti-inflammatory drug an A 3 adenosine receptor agonist that modulates signaling proteins like P13K PKA PKBAkt IKK and NF-κB. Dry eye like glaucoma is often a multitreatment disease especially if the disease is more severe. Now a new device is helping dry eye suffers find relief when eye drops dont work.
Food and Drug Administration to treat cystic fibrosis. Optometrists have several effective instruments to manage their patients. Our algorithm helps provide numerous treatment alternatives to try in those situations.
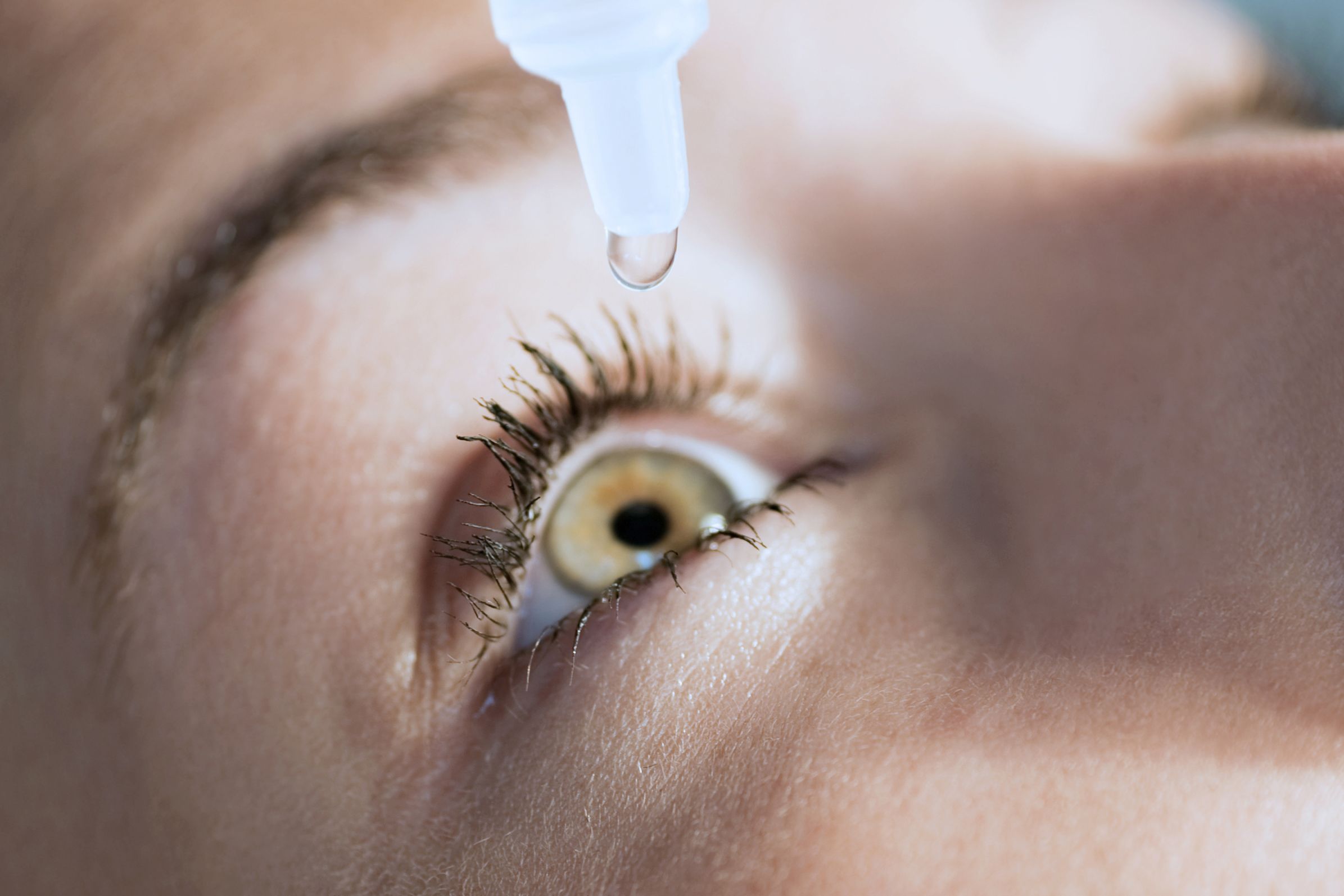
Started his entrepreneurship journey in 2016 after attaining an. Traditionally doctors have battled the condition with dry eye treatments such as drops warm compresses even tiny punctal plugs that fit in the drainage structures of the eyelid to keep lubricating fluids on the surface longer. They may also require maintenance sessions.
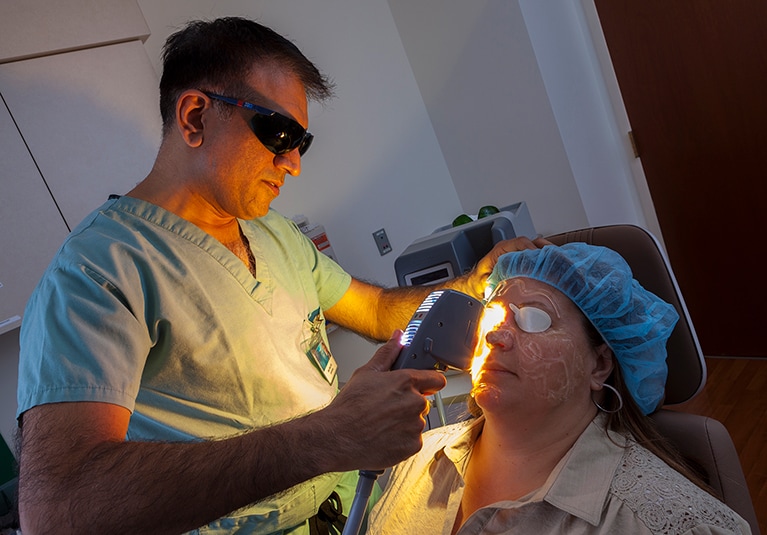
This modulation then inhibits cytokine production. Other recent experimental approaches to dry eye include radio frequency and intense pulsed light therapy IPL said Dr Kaufman.
The biggest news regarding the treatment of dry eye is the potential approval of lifitegrast Shire which is a small-molecule integrin antagonist that inhibits T-cell inflammation by blocking the binding of two key cellular surface proteins LFA-1 and ICAM-1.
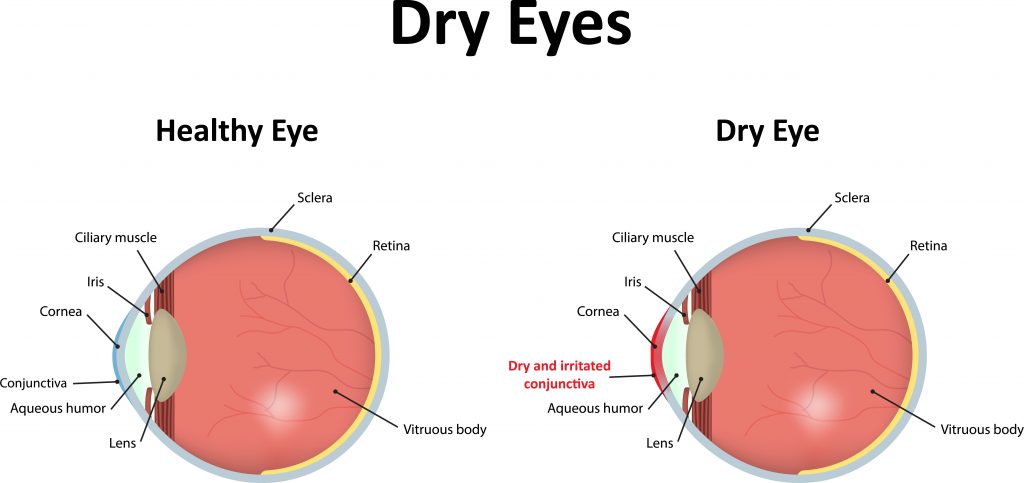
While the proliferation of dry eye pharmaceutical agents has been sluggish the dry eye device market has not. Traditionally doctors have battled the condition with dry eye treatments such as drops warm compresses even tiny punctal plugs that fit in the drainage structures of the eyelid to keep lubricating fluids on the surface longer. Its called TearCare and its a wearable thermal device that goes over the eye lids. While the proliferation of dry eye pharmaceutical agents has been sluggish the dry eye device market has not. Youll find information on Horizon Eye Cares Dry Eye page. Scientists have discovered a potential new treatment for a disease that causes severe dry eyes and dry. Patients may need cyclosporine and lifitegrast and plugs and maybe a topical antibiotic. Cavanaugh Eye Center is now offering Xiidra lifitegrast ophthalmic solution a new treatment approach to chronic Dry Eye Disease DED. Dry eye syndrome is one of the most common disorders of the eye with studies citing anywhere from 8-33 prevalence in the general population.
Can-Fite BioPharma Ltd Petah-Tikva Israel is developing CF101 as an oral drug to treat dry eye. DNase is currently approved by the US. New Dry Eye Treatment. They may also require maintenance sessions. While the proliferation of dry eye pharmaceutical agents has been sluggish the dry eye device market has not. February 5 2020 tearrestore. Recently introduced Xiidra represents a true breakthrough in dry eye treatment.


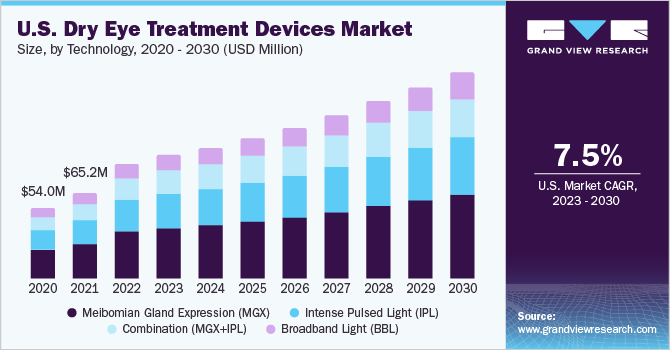











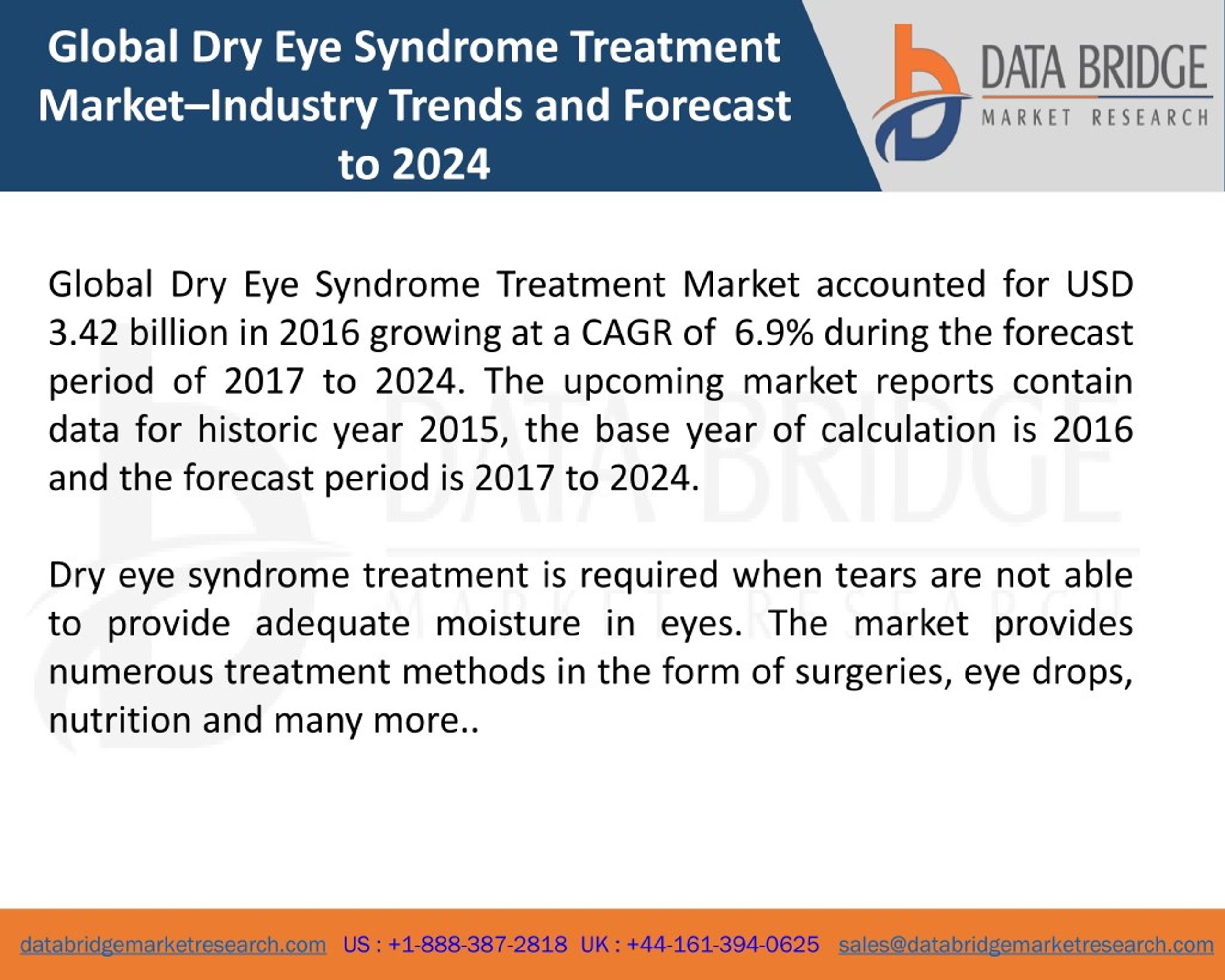
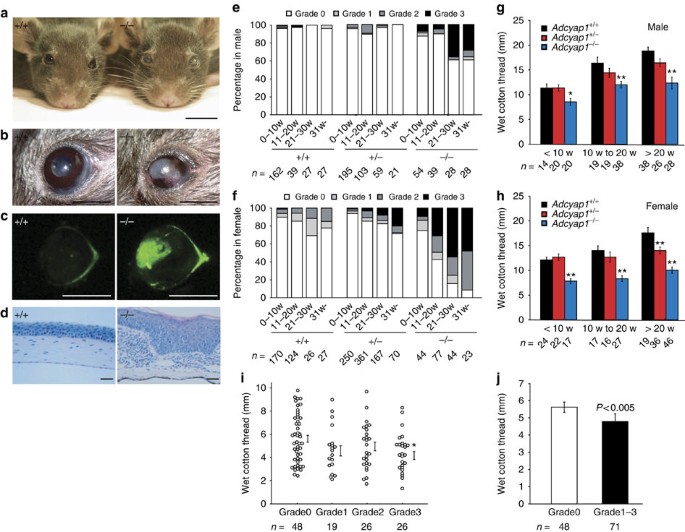






















Post a Comment for "New Dry Eye Treatment 2016"